Stargardt Disease Treatment with Topical Acetylcholinesterase Inhibitor Medication, Low Dose Echothiophate Iodide
Gerard M Nolan, MD September 2021
Abstract
Purpose: This paper presents evidence of improvement of visual acuity and color vision in patients with Stargardt disease achieved by the application of a topical cholinergic medication (TCM), i.e., low dose echothiophate iodide.
Design: Uni-center retrospective study
Participants: Three hundred nine patients with confirmed Stargardt disease
Methods: Three hundred nine participants were followed with 115 participants analyzed over one year. Thirty-one participants were followed for 5 years and 7 for greater than 10 years. Best corrected visual-acuity (BCVA) was measured using the Early Treatment Diabetic Retinopathy Study (ETDRS) protocol. Standardized reporting forms were used to collect participants’ clinical observations. The data was managed using the Excel spreadsheet application.
Main Outcome Measures: Maximum visual improvement (MVI) and improvement at 1 year, 5 years and greater than 10 years; color vision improvement.
Results: Analysis showed a significant MVI. Significant improvement at 1 year, 5 years and greater than 10 years was also realized. The MVI depended on the baseline BCVA as follows:
- Mean BCVA of eyes with baseline BCVA of 20/25 or better improved by 0.11 logMAR (5.5 ETDRS letters)
- Mean BCVA of eyes with baseline BCVA between 20/25 and 20/70 improved by 0.19 logMAR (9.5 ETDRS letters)
- Mean BCVA of eyes with a baseline BCVA between 20/70 and 20/200 improved by 0.22 logMAR (11 ETDRS letters)
- Mean BCVA of eyes with a baseline BCVA between 20/200 and 20/400 improved by 0.27 logMAR (13.5 ETDRS letters)
- Mean BCVA of eyes with a baseline BCVA less than 20/400 improved by 0.39 logMAR (19.5 ETDRS letters)
Color vision testing by Ishihara Color Test (10 plates) improved by a mean of 2.7 plates.
Conclusions: Gains in visual acuity in Stargardt disease patients appear significant and prolonged over time using a TCM, i.e., low dose echothiophate. These improvements are especially effective in patients with baseline BCVA 20/200 to 20/400 and baseline BCVA less than 20/400. There were also improvements in color vision.
Introduction
In 1909, the German ophthalmologist Karl Stargardt described 7 patients from 2 families with a reduction of visual acuity and a normal retinal fundus appearance in young patients during the first or second decade of life. Eventually, these patients developed the classic atrophic macular lesions surrounded by yellowish, deep retina-flecks.1
Today, Stargardt disease is the most common inherited single-gene retinal disease.2 It usually has an autosomal recessive inheritance caused by mutations in the ABCA4 gene. Rarely, it has an autosomal dominant inheritance due to defects with ELOVL4 or PROM1 genes. In Stargardt disease, STGD1, the genetic defect causes malfunction of the ATP-binding cassette transporter (ABCA4) protein of the visual phototransduction cycle.3 Defective ABCA4 leads to improper shuttling of vitamin A throughout the retina and accelerated formation of toxic vitamin A dimers. When vitamin A dimers and byproducts damage the retinal cells, fluorescent granules called lipofuscin appear in the retinal pigmented epithelium of the retina. This is a reflection of such damage.4
Stargardt disease inevitably leads to significant and previously irreversible loss of vision. For example, Progstar Report Number 6, a study of 259 Stargardt disease patients, showed a loss of at least one line of vision in 12.9% of eyes over 1 year.5
In 1959, echothiophate iodide, Phospholine Iodide®, was approved by the Food and Drug Administration (FDA). It has been used over the past 60 years for the treatment of glaucoma and accommodative esotropia. The FDA labeling shows efficacy and safety in both adults and children. In 2007/2008 and 2020 there was temporary shortage of the echothiophate iodide due to demand outstripping supply.
Cholinergic stimulation (the parasympathetic nervous system) uses acetylcholine (ACh) at its receptors and synapses and is regulated by the enzyme acetyl cholinesterase. Echothiophate iodide inhibits this enzyme acetyl cholinesterase and allows for the increase of endogenous acetylcholine. Since 1994, patients have been treated with a TCM (off-label). This included pilocarpine, carbachol and micro-doses of echothiophate iodide, etc. This treatment was initially for refractive eye disease and presbyopia.6 Following success with these two diseases, TCM, i.e., echothiophate iodide, use was expanded to include treatment of macular degeneration7 and Stargardt disease.8,9
Since June 18, 2001, 309 diverse Stargardt Disease patients were observed and treated with off-label micro doses of echothiophate iodide. Eighty-three percent of patients came from within the USA (42 states). The remaining 17% of patients travelled from 5 continents and 24 countries. Unfortunately, many patients were unable to return on a continuing basis due to monetary constraints. The study included patients of many different nationalities and ethnicities. The majority of patients was White, 267 (86.4%) individuals. The non-white group consisted of 42 (13.6%) patients. There were Hispanics, Blacks, Asians and Indians in this group. The patients included very young children, adolescents, adults and senior citizens. The average patient age at baseline was 31.3 years and the age range was 6.8 to 75.2 years. There were 52 (16.8%) patients who also had another family member in the 309-patient baseline. There were 23 sets of siblings including a subset of 3 pairs of identical twins. One family had 3 siblings and a parent in the study. Three parents also were examined and recorded as generational. The database included 139 (45%) females and 170 (55%) males. Table 1 summarizes patient demographics.
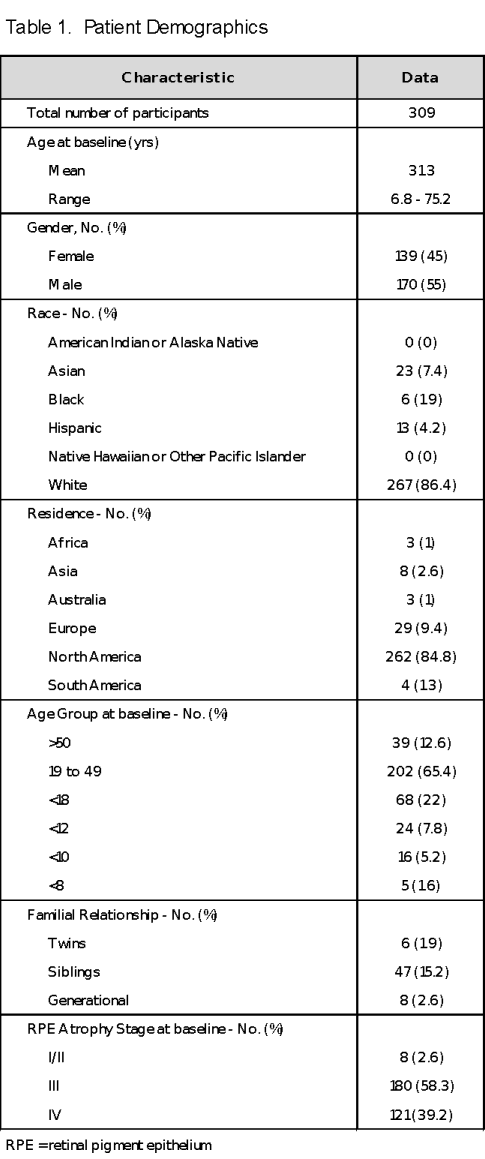
Methods
TCM, micro-doses of echothiophate iodide, were self administered into the lower cul-de-sac just prior to sleep every other day. Patients under the age of 40 were treated at a concentration of 0.015%. Patients over the age of 40 were treated at a concentration of 0.0075%. The current recommended treatment level for glaucoma is 0.125% twice daily. The effective dose of echothiophate for treatment of patients under age 40 years with Stargardt disease is only 3% of the effective dose used for patients with glaucoma. For patients over age 40 years with Stargardt disease, the effective dose is only 1.5% of the effective dose used for glaucoma.
Institutional Review Board (IRB)/Ethics Committee approval was obtained for a one-year study (2006 - 2007) for 22 patients. Two of these patients were followed beyond one year using the same protocols. An additional 289 non-IRB patients, some of whom were treated before implementation of IRB, were studied utilizing the same IRB protocols.
Data for the analysis come from 309 patients including 22 Institutional Review Board (IRB) registered patients.10 Most patients returned for 4 to 6 visits over the subsequent 3 to 6 months. One hundred fifteen patients (22 IRB) continued with treatment and had return visits at one year or later. There were 31 (2 IRB) patients who returned for greater than 5 years. Finally, a group of 7 patients (1 IRB) continued treatment and visited for more than 10 years. Patient 221 is the longest treated at 18 years, 45 visits. Detailed case studies are available for Patients 61, 86, 247 and 260.
Baseline visit best corrected visual acuity (BCVA) was tested for distance vision and near vision, per Early Treatment Diabetic Retinopathy Study (ETDRS) guidelines. Color vision was tested using the Ishihara Color Test (10 plates). Bio microscopy/Slit-lamp examination with tonometry, Computerized Perimetry (CP), Optical Coherence Tomography (OCT), Fundus Photography and Fluorescein Angiogram (FA) were performed. At each visit the work-flow sheet was repeated except for FA which is done only at the initial visit. A standardized clinical report form was used to record the observations. Figure 1 presents the work flow at each visit.

All BCVA measurements post August 2005 were recorded using the logMAR scale. When recording visual acuity using the logMAR chart, each letter has a score value of 0.02 log units. Since there are 5 letters per line, the total score for a line on the logMAR chart represents a change of 0.1 log units. Measurements pre August, 2005 were recorded using the Snellen classification system and converted to the logMAR scale.
Maximum visual improvement (MVI) was calculated by selecting the BCVA from the subsequent visit showing the greatest improvement over baseline. BCVA at 1, 5 and 10 years was the measurement at that milestone in time. To simplify the analysis, the one eye for each patient (309 eyes) showing the most improvement or change was utilized in the study.
Statistical Analysis
BCVA data at baseline, one year, 5 years, >10 years and maximum BCVA were used in this analysis. Patients were categorized per the World Health Organization International Classification of Diseases, tenth revision. The visual impairment categories are as follows:
• Functional | BCVA | ≤ 0.10 logMAR (20/25 or better) |
• Mild | BCVA | 0.12 - 0.54 logMAR (20/25 - 1 to 20/70) |
• Moderate | BCVA | 0.56 - 1.0 logMAR (20/70 - 1 to 20/200) |
• Severe | BCVA | 1.02 - 1.30 logMAR (20/200 - 1 to 20/400) |
• Blind | BCVA | ≥ 1.32 logMAR (less than 20/400) |
FA was used to classify patients by retinal pathology into 4 groups or stages, similar to the Fishman Fundus Flavi Maculatus clinical classification.11
Stage I | Flecks are present locally in the macula and between the temporal arcades, no atrophy of the retinal pigment epithelium (RPE). |
Stage II | Flecks are diffusely present, temporally in macula, but also above or below the temporal arcade, and/or nasally, no atrophy of the RPE. |
Stage III | Localized disease as in stage I, RPE Atrophy. |
Stage IV | Diffuse disease as in stage II, RPE Atrophy. |
For all analysis, ETDRS letter scores were converted to the logMAR scale. The baseline data of the 309 participants were measured at distance, near and color vision. Baseline demographics were generated by gender and age. Baseline RPE atrophy stage and World Health Organization (WHO) classification of visual impairment were generated. The results were evaluated to show correlation between visual acuity and RPE atrophy. Color vision impairment data were collected using Ishihara Color Test (10 plates).
One-year data from 115 patients (22 IRB), BCVA at distance and near, was collected and compared to baseline. Distance and near BCVA data at 5 years was collected from 31 patients (2 IRB) and compared to baseline. Data obtained over 10 or more years was collected from 7 patients (1 IRB). The mean BCVA and most-recent-visit BCVA were tabulated. Also, BCVA distance and near data was compared to baseline.
Table 2 defines patient categories.
Results
The All-patient group consisted of 309 patients (22 IRB). The mean age was 31.3 with a range of 6.8 to 75.2 years. There were 8 (2.6%) patients classified as RPE atrophy: stage I/II at baseline. When reviewing the fundus photography of these patients, they did not show evidence of retinal pathology or abnormality, i.e., deep retinal flecks (similar to Dr. Stargardt’s presentation of 1909). Patient 86 (IRB) presented at baseline with non-atrophic disease (RPE atrophy: stage I/II) in 2006. By 2008 the patient had developed diffuse atrophic disease (RPE atrophy: stage IV).
One hundred eighty (57.7%) patients presented at baseline with atrophic retinal disease localized inside the temporal arcade were classified as RPE atrophy: stage III. One hundred twenty-one (39.7%) patients had diffuse retina atrophy above and below the temporal arcades and on the nasal side of the retina were classified as RPE atrophy: stage IV.
At baseline, distance BCVA mean value of the all-patients group was 0.96 logMAR. Table 3 shows the BCVA mean value and the distribution of patients over visual impairment categories at each RPE atrophy stage.
For RPE atrophy stage I/II patients, 100% are classified as visual impairment category: moderate or better. For RPE atrophy stage III patients, 37% have a BCVA greater than logMAR 1.00 (20/200) with 8% of those patients classified as visual impairment category: blind and 29% as visual impairment category: severe. For RPE atrophy stage IV patients 61% had BCVA greater than logMAR 1.00 (20/200) with 25% of those patients classified as visual impairment category: blind and 36% classified as visual impairment category: severe.
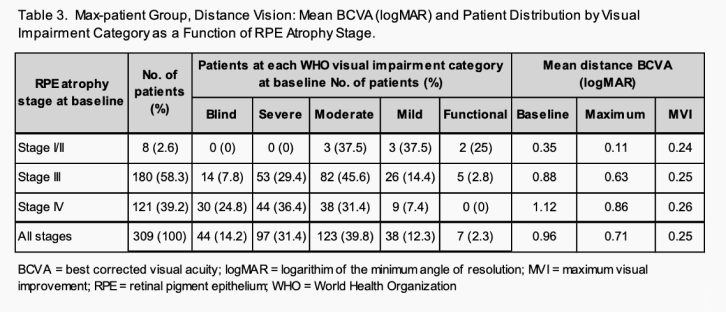
Clinical examination revealed that 2.6% of patients had no overt RPE pigment atrophy. There was RPE atrophy within the temporal arcades in 97.4% of eyes present. Additionally, 39.2% of patients had RPE atrophy outside the arcades. Most patients had flecks present with the exception of the 8 patients in RPE atrophy stage I/II who showed no evidence of retinal flecks. Patient 86 presented in 2006 with RPE atrophy stage I/II with no overt atrophic RPE disease. By 2010 the patient had an inevitable progression to RPE atrophy stage IV.
The MVI was calculated by comparing the baseline BCVA to the best BCVA after the initial visit. Patients generally exhibit visual acuity improvement after initial treatment with maximum improvement at 4 to 6 weeks. The mean maximum BCVA improvement for the Max-patient Group was 0.25 logMAR.
When investigating the mean distance MVI versus anatomical RPE atrophy staging, there was no statistical difference amongst the RPE atrophy stages.
Figure 2 shows the distance and near vision mean MVI for each visual impairment classification.
For distance vision the visual impairment blind and severe categories show clinically significant mean MVI, 0.39 logMAR and 0.27 logMAR respectively. For near vision the visual impairment blind, severe and moderate categories show clinically significant mean MVI. Table 4 shows the distribution of patients as a function of WHO visual impairment category: at baseline and at MVI. The number of patients classified as visual impairment categories blind and severe was reduced from 141 at baseline to 49 at MVI.
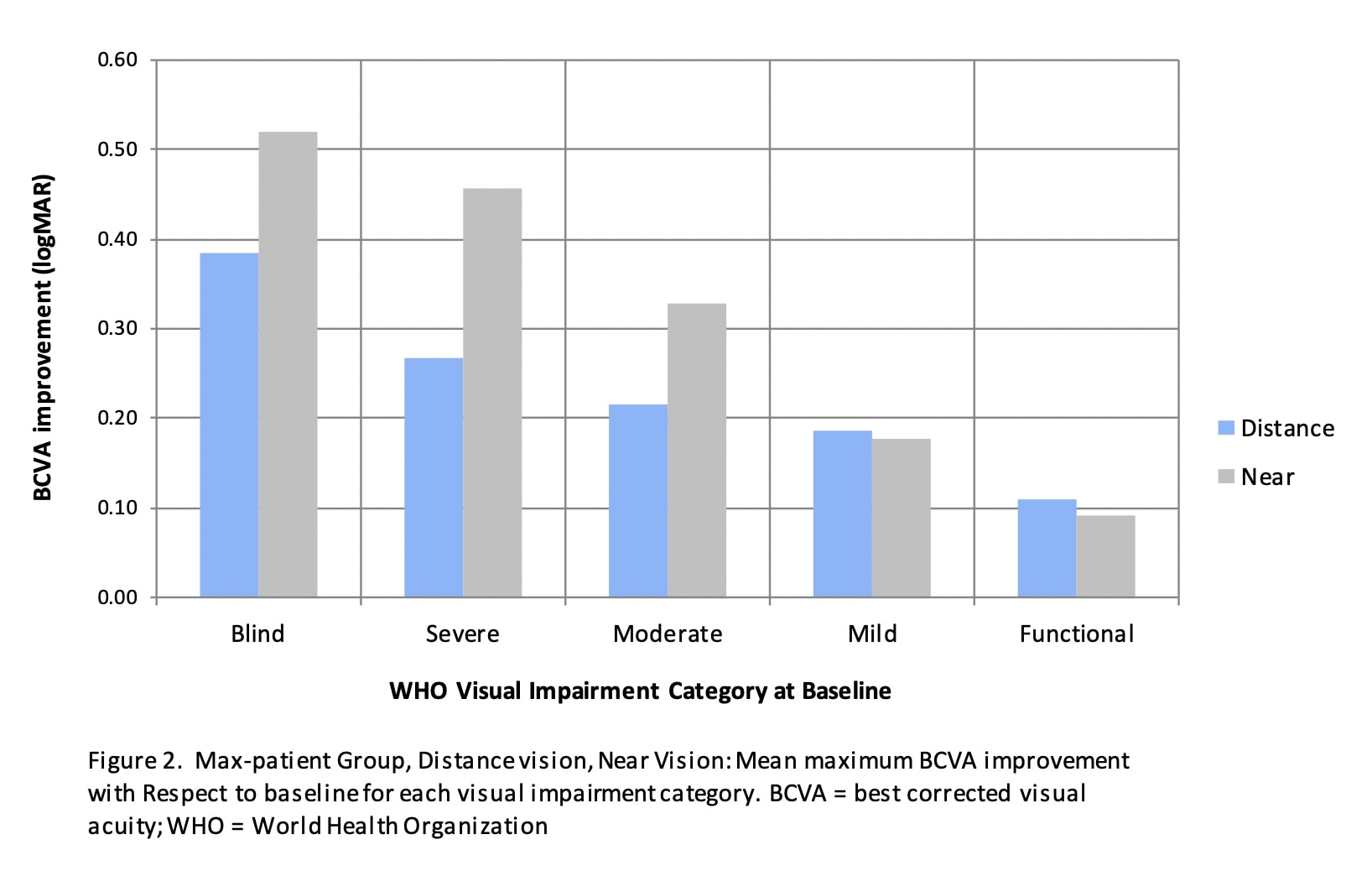
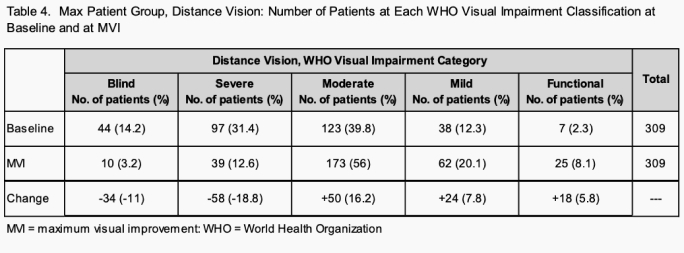
Patients at each visual impairment category at baseline and Max show this statistical effect in the visual impairment blind and severe categories with a reduction of patients from 14% to 3%, and 31% to 13% respectively.
Analysis at one year
The One-year patient group included 115 patients (22 IRB) with a mean age of 29.1 and a range of 6.8 to 72.2 years. Like the Max-patient group, the visual impairment blind and severe categories show the greatest BCVA improvement, 0.29 logMAR and 0.23 logMAR respectively. At one year, significant near vision mean BCVA improvements for the visual impairment blind, severe, and moderate categories were realized.
Analysis at 5 years
The Five-year patient group included 31 patients (2 IRB) with a mean age at baseline of 32.8 and a range of 6.8 to 69.3 years. Similar to the Max-patient group and One-year patient group, the Five-year patient group at 1 year shows the greatest improvement for patients in the visual impairment blind and severe categories.
The visual impairment blind and severe categories show a sustained distance BCVA improvement over 5 years. There were sustained and significant near BCVA improvements for the visual impairment blind, severe and moderate categories. The visual impairment mild and functional categories show a small decline of BCVA with respect to baseline.
Table 5 illustrates the mean BCVA improvement with respect to baseline for the One-year patient group and the Five-year patient group at each visual impairment category:
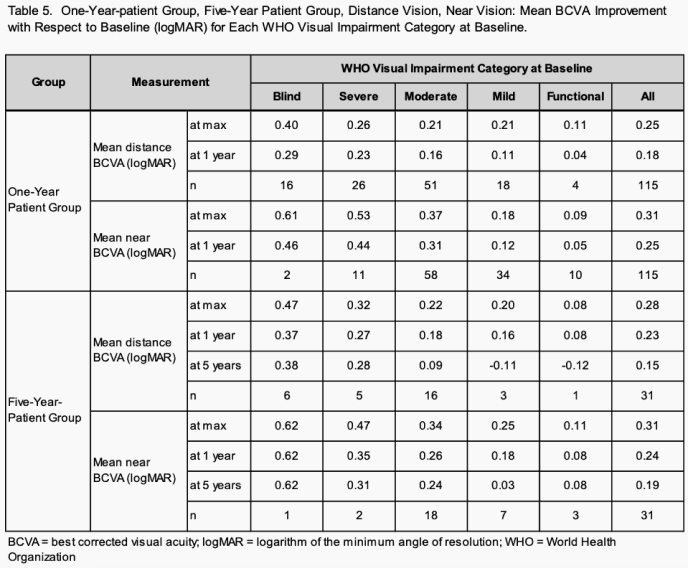
Long Term Patient Analysis
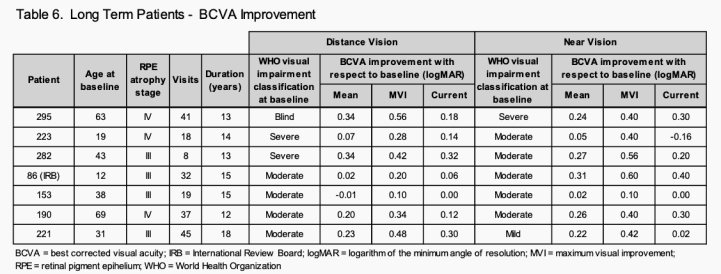
There are 7 patients (1 IRB) in the Long-term patient group. Ages range from 12 to 69 years at baseline with a mean of 36.5. Visits range from 8 to 45 with a mean of 28.6 visits. The mean treatment span was 14.3 years with Patient 221, at 18 years, the longest. Table 6 details the mean BCVA improvement, MVI and current visit BCVA for distance vision and near vision.
Patient 86 (RPE atrophy: stage IV, visual impairment category: moderate) had an additional 0.30 logMAR near vision improvement which was very helpful in allowing him to complete his college studies. Patient 153 (RPE atrophy: stage III, visual impairment category: moderate) had a mean improvement of -0.01 logMAR over 15 years.
Shortage of Echothiophate Iodide
Six-to-8 month shortages of echothiophate iodide occurred during 2007/2008 and 2020 resulting in patients being unable to continue their treatment protocol. All patients under treatment were affected. By the end of the second week without treatment, visual acuity reverted to baseline or worse. The shortages especially impacted patients in the visual impairment blind and severe categories.
Upon resumption of treatment most patients regained BCVA prior to the cessation of treatment. However, some patients did not. The examples below show the effect of cessation and resumption of treatment for 3 patients.
Patient 295 (RPE atrophy: stage IV, visual impairment category: blind) went off medication during the 12th year of treatment. The patient reverted to baseline (1.66 logMAR, less than 20/800 vision), and lost independence. The patient had to navigate slowly with the use of a blind-cane. This was attributed to being off medication for 6 months during the 2020 shortage. Fortunately, several lines of vision returned upon resumption of treatment. This resulted in an improvement of 0.18 logMAR with respect to baseline.
Patient 148 (RPE atrophy: stage III, visual impairment category: mild) went off medication during the fifth year of treatment. The patient had an initial 0.12 logMAR BCVA improvement for the first 4 years of treatment but showed a decline of 0.38 logMAR from baseline in the fifth year. This was attributed to being off-medication for 6 months during the 2007/2008 shortage. Upon resumption of treatment Patient 148 did not regain the initial improvement and remained at this level for the remainder of treatment.
Similarly, as a result of the shortage of 2007/2008, patient 153 (RPE atrophy: stage III, visual impairment category: moderate) went off medication during the third year of treatment. The patient lost the BCVA improvement of 0.10 logMAR and upon resumption of treatment remained at baseline for the next 10 years.
Color Vision Analysis
Color vision was tested using the Ishihara Color Test (10 plates). Color vision loss has previously been reported in Stargardt disease patients.12,13
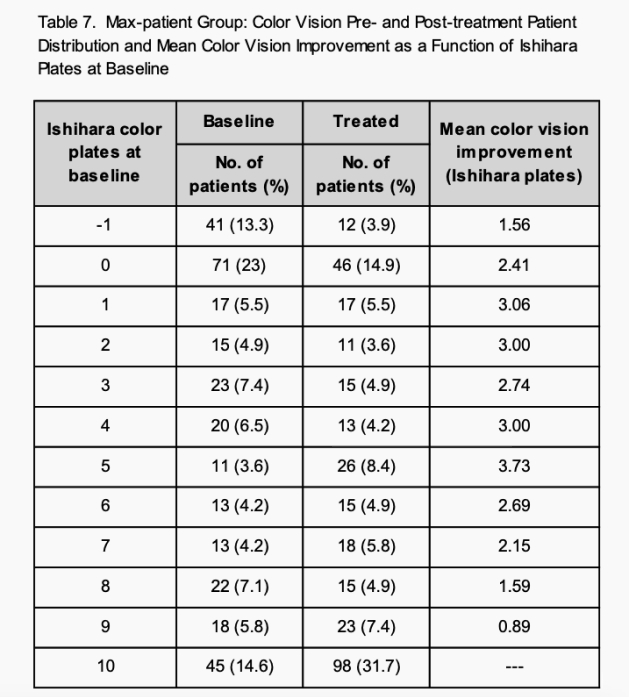
This study revealed an 85% incidence of color vision deficiency at baseline. Table 7 shows the color vision patient distribution pre and post treatment and mean color vision improvement as a function of baseline color vision. Forty-five patients (14.6%) had scores of 10/10 or normal color vision. There were 71 patients (23.0%) who scored 0/10 and another 41 patients (13.3%) who scored -1/10 (as they missed all 10 plates and also the control plate). The baseline mean was 3.6 plates with a median of 3.0 plates.
Substantial improvement was realized post-treatment. After treatment, the number of patients scoring 10/10 plates improved from 45 (14.6%) at baseline to 98 (31.4%). The patients scoring -1/10 decreased from 41 (13.3%) at baseline to 12 (3.9%). The patients scoring 0/10, decreased from 71 (23.0%) at baseline to 46 (14.9%). Post treatment, mean color vision was 5.8 plates, with a median of 6.0 plates. This is an improvement of 2.7 and 3.0 plates respectively.
Category 5 showed the most gain at +3.73 plates. Category 0 added +2.41 plates and categories 9 and 8, which only had a potential of +1 and +2 plates, respectively scored +0.89 and +1.59 plates.
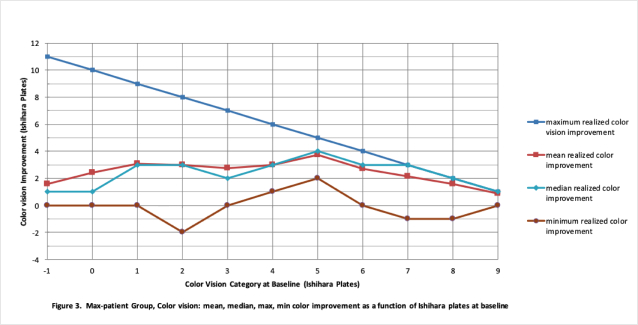
Unexpectedly in June 2001, Patient 260 (the first patient included in this study) had substantial color vision improvement from zero plates to 10 plates.9 Similarly, in March 2011, Patient 227 went from -1 plate to 10 plates for a total of improvement of 11 plates. Figure 3 shows the mean/median and maximum/minimum at each Ishihara Color Test score. In all categories at least one patient achieved the maximum score of 10 plates.
IRB Analysis
The IRB patient group consisted of 22 patients enrolled in the prospective IRB study.10 Please note that the initial 23 patients registered as IRB was reduced to 22 as one patient elected to withdraw from the study due to monetary constraints. The one-year patient evaluation consisted of 9 visits during 2006/2007. The age range at baseline was 12.4 to 40.2 years with a mean of 28.2 years
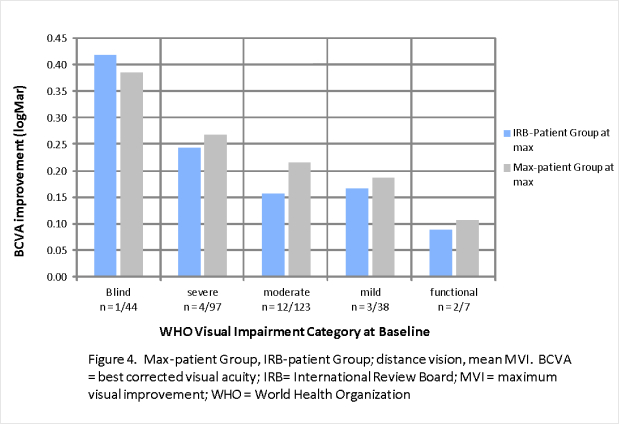
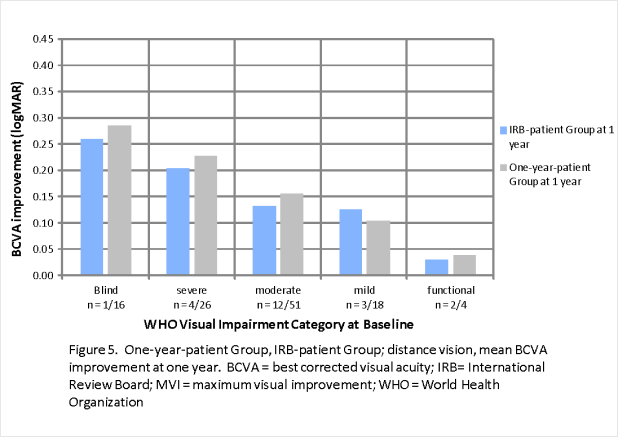
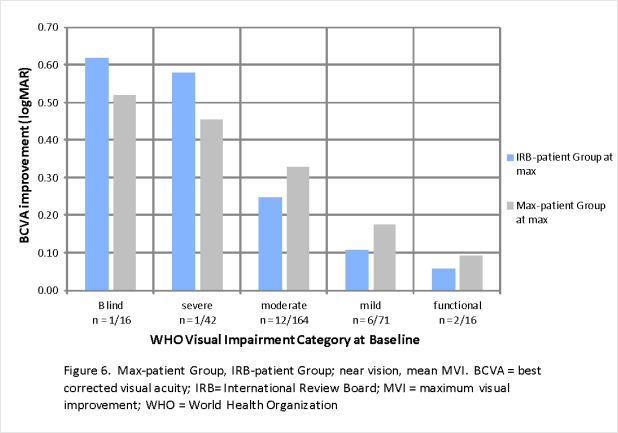
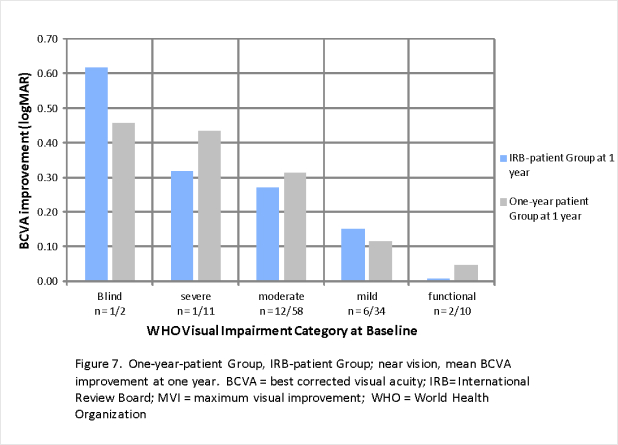
Figure 4 shows good correlation of distance MVI between the IRB-patient group and the Max-patient group. Figure 5 also shows good correlation between the IRB-patient Group and the One-year Patient group at one year. Figures 6 and 7 show similar correlations for near vision improvement.
The near BCVA improvements are all achieved in non-presbyopia patients, under age 40. These gains are considered a low-vision enhancement.
Discussion
This twenty-year uni-center retrospective study shows the demographic, clinical characteristics, BCVA improvement and color vision improvement in the Max, One-year, Five-year, and Long-term patient groups. The study presents evidence of an overall improvement for all patient groups with the magnitude of BCVA improvement especially significant for patients in the visual impairment blind and severe categories. Only one patient (RPE atrophy: stage IV; visual impairment category: mild) from the IRB group showed a loss of at least one line of vision.
These findings compare favorably with the natural history of Stargardt disease patients reported in the Progstar Report Number 65 which shows a loss of at least one line of vision in 12.9% of eyes and a rate of loss which differed depending upon baseline BCVA. In the Progstar study, the BCVA of eyes with baseline BCVA of 20/25 or better declined 2.8 ETDRS letters at one year. Eyes with baseline BCVA between 20/25 and 20/70 declined 2.3 ETDRS letters. Eyes with baseline BCVA between 20/70 and 20/200 declined 0.8 ETDRS letters. Eyes with baseline BCVA worse than 20/200 showed an improvement of 2.3 ETDRS letters.
Due to reported natural degradation of BCVA for most Stargardt disease patients over time, stabilization of vision at baseline levels, can be considered an improvement over the natural progression of this disease.
This study revealed a correlation between RPE atrophy and baseline BCVA. Greater levels of atrophy correlated with lower visual acuity. For example, the prevalence of patients at RPE atrophy: stage IV classified as WHO visual impairment category: blind was 3 times of those at RPE atrophy: stage III. Previous studies did not report this finding. This may be a result of limiting patient inclusion to those with a BCVA at 20/400 or better.
BCVA is the most common outcome measure for efficacy studies of retinal diseases.14 In clinical trials a gain of 0.3 logMAR or 15 ETDRS letters (equivalent to 3 Snellen lines) is considered clinically significant. Many patients in the visual impairment blind and severe categories achieved this milestone for distance BCVA improvement. Additionally, for near vision, there were patients in the visual impairment moderate category who achieved 0.3 logMAR or 3 lines of improvement.
BCVA is an important visual function outcome directly related to participants' daily activities.15 Individuals with vision impairment are more likely to experience restrictions in their independence, mobility and educational achievement. They also have an increased risk of falls, fractures, injuries, poor mental health, cognitive deficits and social isolation. The patients in this study reported improvement in maintaining independence and in performing the activities of daily living such as cooking, shopping, watching television, reading and personal hygiene. Some have been able to complete college, remain employed and obtain restricted driver’s licenses.
Likewise, restoration of partial to nearly complete color vision in some patients not only improves enjoyment of the color environment but adds to the safety for both the individuals and their communities
The exact mechanism of action of echothiophate iodide in Stargardt disease patients is not yet known. Perhaps, dilute echothiophate iodide makes endogenous ACh more available to diseased neuroreceptor populations across the retina. Increased ACh levels may amplify the synaptic potential of the surviving photoreceptors and ganglion cells, making it possible for these reduced populations to achieve threshold and resume the propagation of visual information to the brain. This effect would parallel previously described mechanisms of drug action in other neurological diseases, such as Parkinsonism, Alzheimer’s Disease and Clinical Depression.
Stargardt disease is one of a number of degenerative retinal diseases, including dry age-related macular degeneration7 and retinitis pigmentosa, which may respond to TCM therapy. If this turns out to be the case, this therapy may hold the potential to lessen the disability from a number of blinding retinal diseases.
In summary, this study presents evidence of BCVA and color vision improvement in Stargardt disease patients by the application of a topical cholinergic medication, i.e., low dose echothiophate Iodide, over a period of many years. Patients with the poorest BCVA at baseline had significant statistical improvement in BCVA.
References
- K. B. Stargardt 1909 (), "Über familiäre, progressive Degeneration in deer Makulagegend des Auges", Albrecht von Graefes Archiv fürp[ Ophthalmologie (in German), 71(3), pp. 534–550, doi:10.1007/BF01961301, S2CID 12557316
- Michaelids M, Hunt DM, Moore AT. The genetics of inherited macular dystrophies. J Med Genet. 2003;40(9):641-650.
- Ikmets R, Singh N, Sun H, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated ub recessive Stargardt macular dystrophy. Nat Genet. 1997;15(3):236-246.
- Adler L, 4th; Boyer, NP; Chen, C; Ablonczy, Z; Crouch, RK; Koutalos, Y (2015). The 11-cis Retinal Origins of Lipofuscin in the Retina. Progress in Molecular Biology and Translational Science. 134. pp. e1–12. doi:10.1016/bs.pmbts.2015.07.022. ISBN 9780128010594. PMID 26310175.
- Kong X, Straus R, et al, Visual Acuity change over 12 months in the prospective progression of Atrophy secondary to Stargardt Disease ProgSar Report Number 6, Ophthalology 2107 Nov (11)1640-1651
- Nolan G, inventor; Methods for treating various eye disorders. US Patent 6,273,092 B1. August 14, 2001 (filed September 22, 2000).
- Nolan G, inventor; Method of treating certain eye diseases. US Patent 6,605,640 B2. August 12, 2003 (filed January 31, 2001).
- Nolan G, inventor; Physiological method of improving vision. US Patent 6,540,990 B2. April 1, 2003 (filed August 13,2001).
- Nolan G, inventor; Physiological method of improving vision. US Patent 7,915,312 B2. March 29, 2011 (filed March 19, 2003).
- IRB file number – 05175-01 (November 23, 2005) A Pilot, open-label study of low dose ocular echothiophate iodide for the treatment of Stargardt’s [sic] Disease. Principal Investigator Number - 5575-001. Sponsor - Independent Review Consulting, Inc. (IRC), Corte Medera, California
- Fishman G, Fundus Flavimaculatus, A clinical classification. Arch Ophtalmol. 1976; 94(12): 2061-2067.
- Mantyjarvi M, Tuppurainen K. Color Vision in Stargardt Disease, Int. Ophthalmol 1992 Nov,16(6):423-8 doi:10.1007
- Leroy BP, Breusegem, C.M. et al. Colour vision Deficiency in Stargardt Disease. IOVS May 2008, Vol 9, 3826.doi
- Beck RW, Magurie MG, Bressler NM, et al. Visual acuity as an outcome measure in clinical trials of retinal diseases. Ophthalology. 2007;114(10):1804-1809.
- Szlyk JP, Fishman GA, Grover S, et al. Difficulty in performing everyday activities in patients with juvenile macular dystrophies” comparison with patients with retinitis pigmentosa. Br J Ophthalmol, 1998;82(12):1372-1376.
Abbreviations and Acronyms:
BCVA = best corrected visual acuity; CP = Computerized Perimetry; logMAR = logarithm of the minimum angle of resolution; ETDRS = Early Treatment Diabetic Retinopathy Study; FA = Fluorescein Angiogram; FDA = Food and Drug Administration; IRB = Institutional Review Board; MVI = maximum visual improvement; OCT = Optical Coherence Tomography; RPE = retinal pigment epithelium; TCM = topical cholinergic medication; WHO = World Health Organization
 Return home
Return home