Stargardt Disease Treatment with Topical Acetylcholinesterase Inhibitor Medication, Low Dose Echothiophate Iodide, Patient 260
Gerard M Nolan, MD September 2021
Abstract
Purpose
To demonstrate visual acuity and color vision improvement realized by a Stargardt disease patient resulting from topical cholinergic medication (TCM).
Observations
Early and sustained improvement in both visual acuity and color vision in a patient with Stargardt disease. The improvement was a result of topical application of low dose echothiophate iodide (0.015%). The gains in visual acuity and color vision have been maintained through a period of observation of more than 5 years.
Conclusions and Importance
TCM, low dose echothiophate iodide therapy produced significant and sustained gains in BCVA and color vision for this patient. This compares favorably to the natural degradation to legal blindness in untreated Stargardt disease patients. Low dose echothiophate iodide therapy potentially provides a treatment for Stargardt disease where none was previously available.
Introduction
Today, Stargardt disease is the most common inherited single-gene retinal disease. It usually has an autosomal recessive inheritance caused by mutations in the ABCA4 gene.1 Stargardt disease inevitably leads to significant and irreversible loss of vision. Cholinergic stimulation (the parasympathetic nervous system) uses acetylcholine (ACh) at its receptors and synapses and is regulated by the enzyme acetyl cholinesterase. Echothiophate iodide inhibits this enzyme acetyl cholinesterase and allows for the increase of endogenous acetylcholine.
This case history presents evidence of early and sustained improvement in visual acuity and color vision in a Stargardt Disease patient. The improvement was a result of the application of TCM, low dose echothiophate iodide (0.015%).
Case Report / Findings
The present case involves a female patient diagnosed with Stargardt disease at age 19. This individual presented with no familial history of blindness or retinal disease. The patient took no medication other than birth control pills.
With the exception of a mild astigmatism, this patient was normally sighted through age 17, at which time the patient began noticing difficulty distinguishing between whiteboard marker colors in high school classes. Visual acuity continued a mild decline until age 19, at which point the patient experienced an abrupt drop in both central vision and night vision. The patient consulted with several retinal specialists at university hospitals including The Johns Hopkins Wilmer Eye Institute; each specialist confirmed a diagnosis of Stargardt disease.
Treatment Protocol
The patient initially presented for baseline testing at age 25 on June 18, 2001. This patient is the initial case report of a 309 Stargardt disease patient study. The treatment protocol was derived from recent observations of clinical ophthalmic research during the prior 24 months.
Prior diagnosis of Stargardt disease was verified via fluorescein-based angiogram. Distance best corrected visual acuity (BCVA) was established using the Snellen test, near BCVA by the Rosenbaum test and color vision by the Ishihara 10 plate test. All measurements were converted to logMAR for analysis. Pupil constriction and corrective prescriptions were noted. The fluorescein-based angiogram showed moderate RPE dropout localized to the macula. The patient was classified as RPE Atrophy Stage III. World Health Organization (WHO) visual impairment category was blind in the right eye and severe in the left for distance vision. For near vision it was moderate for both eyes.
The patient’s right eye was selected as the initial target eye. The patient was instructed in the proper self-administration of eye drops, then further instructed to self-administer just one drop of dilute (0.015%) echothiophate iodide to the target eye, immediately prior to receiving eight hours of uninterrupted sleep. The initial dosage used for treatment of this patient was 0.015%, administered to each eye once every four days, immediately prior to sleep, thus minimizing runoff due to eye movement and blinking. The source for the eye drops was Wyeth-Ayerst, Philadelphia, PA. The product, Phospholine Iodide®, included both echothiophate iodide and its buffered diluent.
The following day, the patient returned for retesting of visual acuity. The patient was instructed to treat the left eye that evening, then to begin a regimen of treating alternating eyes every fourth night. Within the first month, the frequency was increased to every second evening. Each eye, therefore, received one drop of 0.015% echothiophate iodide every four days. Follow-up fluorescein-based angiograms and acuity tests were performed at six-month intervals.
Results
The pre-treatment examination determined the patient's right eye BCVA to be 5/400 (1.9 logMAR) and left eye BCVA 20/300 (1.2 logMAR), eccentric fixation. Both eyes near BCVA was 20/200 (1.00 logMAR). The patient’s gaze was eccentric with particular difficulty in near vision focus. Color vision was contrast-only, zero plates in the right eye and 1 plate in the left.
The morning following initial echothiophate iodide administration, the patient reported a mild dimming of vision in the target (right) eye upon awakening. The patient experienced little subjective change in vision until nearly an hour after waking, at which time the patient could see colors in the hotel room not discernible the day before. Upon looking out the window, the patient saw traffic passing 200 yards away and could call out the colors of individual cars as they passed.
Examination 24 hours after initial treatment revealed characteristic pupil constriction. The patient's right eye distance BCVA had improved to 20/300+1 (1.18 logMAR). The left eye remained unchanged at 20/300 (1.20 logMAR). Near BCVA improved to 20/25 (0.10 logMAR) both eyes. Color vision improved from 0 to 4 plates in the treated eye and from 1 to 10 in the untreated eye.
Subjective complaints during the first several weeks of therapy included mild lid twitching, transient dimming and blurring of vision on the morning following administration. Mild vision-related headaches were also reported but did not discourage the patient from continuing treatment. These side effects were transient and limited to the first two months. Pupil constriction was present upon awakening following day administration and was present for several hours. The patient considered this beneficial as the pinhole vision resulted in a boost of visual clarity. None of the complaints of ocular burning, lacrimation, brow-ache or lens opacities associated with the traditional 0.25% protocol of echothiophate iodide treatment have been reported by this patient.
After 4 months of therapy, the patient’s BCVA reached an initial plateau of 20/200+1 (0.98 logMAR) for distance and 20/25 (0.10 logMAR) for near. Color vision improved to 10 plates in both eyes. The improvement has been maintained for more than 5 years. The patient currently wears glasses for distance only (-1.25 diopter). Table 1 shows the patient’s BCVA and color vision history.
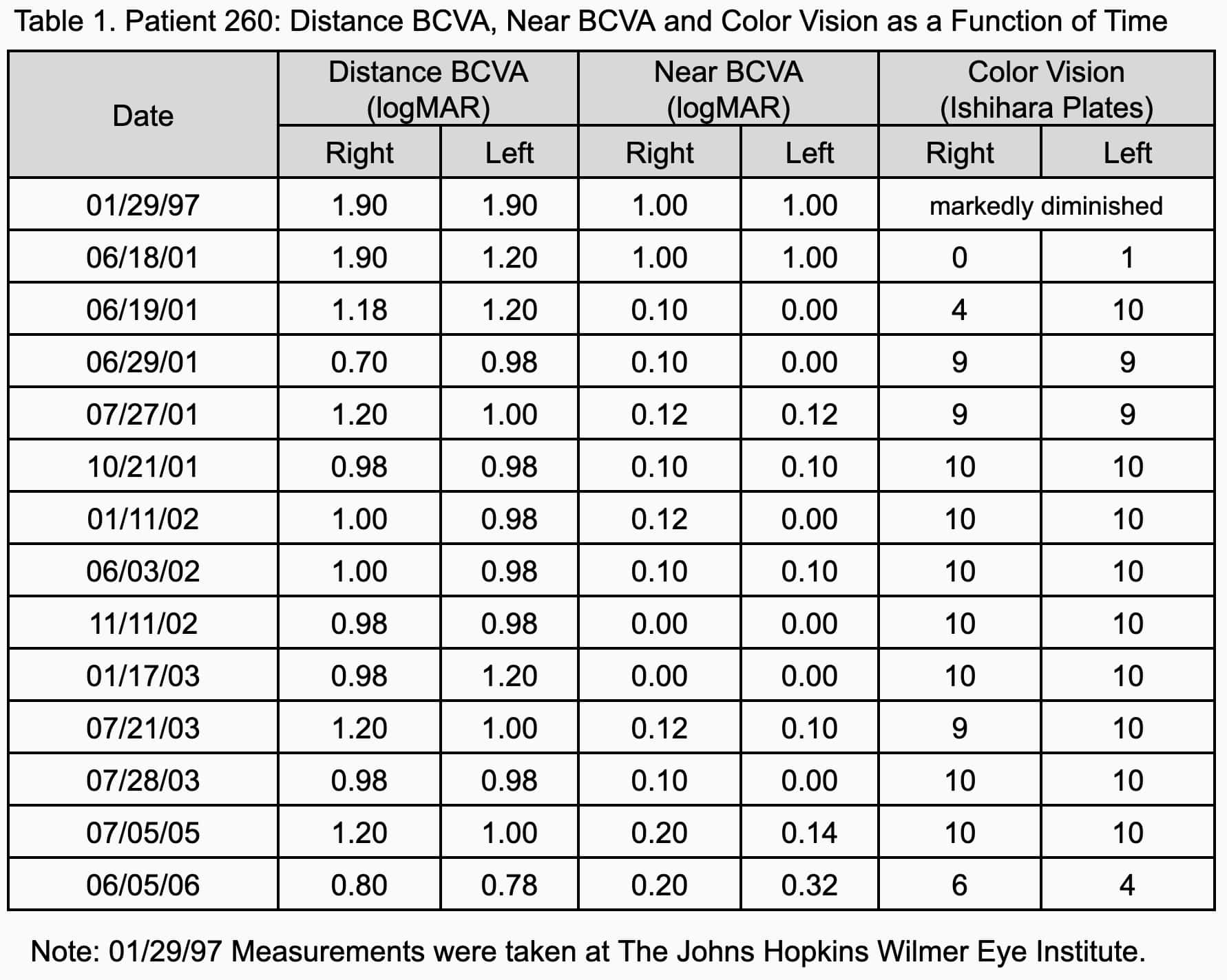
The patient went without echothiophate iodide therapy for two weeks during the 5 year treatment period. This was due to the patient’s failure to pack medication for her honeymoon. After five days without echothiophate iodide, the patient’s vision dropped back to legal blindness. The patient also lost color vision. However, visual acuity and color vision improved and stabilized upon the resumption of medication.
Discussion
In this patient’s case, dilute echothiophate iodide has provided an effective, non-surgical therapy. The patient was legally blind, as validated by several leading institutes. The patient was unable to navigate without the assistance of a cane or guide dog. The patient could not read or write a grocery list, keep a job or watch television. The vision gains have restored the patient’s independence and the patient returned to the workforce. During 5 years of successful therapy, no local or systemic side effects were noted. No tolerance or decline in drug efficacy was observed.
In summary, this case study presents evidence of BCVA and color vision improvement for this Stargardt disease patient resulting from the application of low dose echothiophate. The treatment resulted in a marked improvement of quality-of-life.
Conclusions
Stargardt disease is one of a number of degenerative retinal diseases, including dry age-related macular degeneration and retinitis pigmentosa, which may respond to TCM therapy. If this turns out to be the case, this therapy may hold the potential to lessen the disability from a number of blinding retinal diseases.
The exact mechanism of action of echothiophate iodide in Stargardt disease patients is not yet known. Perhaps, dilute echothiophate iodide makes endogenous ACh more available to diseased neuroreceptor populations across the retina. Increased ACh levels may amplify the synaptic potential of the surviving photoreceptors and ganglion cells, making it possible for these reduced populations to achieve threshold and resume the propagation of visual information to the brain. This effect would parallel previously described mechanisms of drug action in other neurological diseases, such as Parkinsonism, Alzheimer’s Disease and Clinical Depression.
Topical cholinergic medications, echothiophate iodide, pilocarpine, etc., have been a staple to treat glaucoma and strabismus. Recently, the use of several different cholinergic therapeutics has been expanded to include treatment of presbyopia. This case study illustrates a single Stargardt disease patient treated with a cholinergic medication, i.e., low dose (0.015%) echothiophate iodide. The patient showed initial and long term improvement which has been maintained through a period of observation of more than 5 years. The treatment resulted in a marked improvement of the patient’s quality-of-life.
This compares favorably to the natural degradation of visual acuity often leading to legal blindness caused by this untreatable disease. Hopefully, the ophthalmology community will become aware of this Stargardt disease treatment regimen and add it to their tools for the treatment of this disease.
Patient consent
Written informed consent was obtained from the patient for publication of this case report. This report does not contain any personal identifying information.
Notes
- Michaelids M, Hunt DM, Moore AT. The genetics of inherited macular dystrophies. J Med Genet. 2003;40(9):641-650.
Abbreviations and Acronyms:
ETDRS = Early Treatment Diabetic Retinopathy Study; IRB = International Review Board; RPE = retinal pigment epithelium; TCM = topical cholinergic medication; WHO = World Health Organization
 Return home
Return home